EIQ signs reseller agreement with US based SARC MedIQ
Our AI heart disease detection Investment EchoIQ (ASX: EIQ) just signed a reseller agreement for its FDA cleared Aortic Stenosis detection technology…
Aortic stenosis is a disease where the heart’s aortic valve is narrowed and doesn’t open fully. This restricts blood flow to the rest of the body - it can be fatal if left untreated.
EIQ’s technology plugs into existing imaging processes and helps detect more cases of Aortic Stensosis that would otherwise be missed by clinician-only imaging processes’.
EIQ is also developing heart failure detection tech, which today’s announcement also impacts (more on why later).
Today, EIQ signed a reseller agreement with SARC MedIQ - a leading provider of imaging workflow solutions in the US…
(Servicing >300 US healthcare facilities and clinics catering to more than 1,500 physicians)
The agreement means EIQ gets immediate access to this network of hospitals/clinics AND all of the sales/distribution capabilities of SARC…
EIQ confirmed that SARC’s sales force was currently being trained and that once distribution is underway it would be receiving a “fee per scan from hospitals and clinics in the SARC MedIQ network”.
Once fully integrated into SARC’s workflows, that could mean EIQ generates revenues on scans across >300 US hospitals/clinics.

Today’s deal builds on EIQ’s previous agreements signed with ScImage and MedAxiom for integration into 36 hospitals and cardiology practices across the US.
The major takeaway for us here is that EIQ is managing to sign multiple deals with multiple partners which we hope means faster integration into workflows across the US.
Integrations is the key metric we are tracking for EIQ in the short term.
Integrations are just another way of saying how many cardiologists are committing to using EIQ’s tech in their scanning process’.
The more integrations, means EIQ’s tech is used more often and could eventually lead to more revenues for EIQ…
Integrations are very important because once EIQ’s tech has been put into place in a clinical setting, it means any future technology EIQ develops can be rolled out almost instantly.
That’s where EIQ’s Heart Failure detection tech comes into play.
All of the integration work EIQ is doing now means EIQ can instantly roll out HF detection tech to those same networks once the FDA clearance comes in…
Fingers crossed today’s deal is the first of many we see come in between now and the end of the year.
Here is EIQ’s CEO (Dustin Haines) take on EIQ’s integration pipeline - note he mentions additional 40-50 hospitals/clinics are in discussions or are evaluating EIQ’s tech:
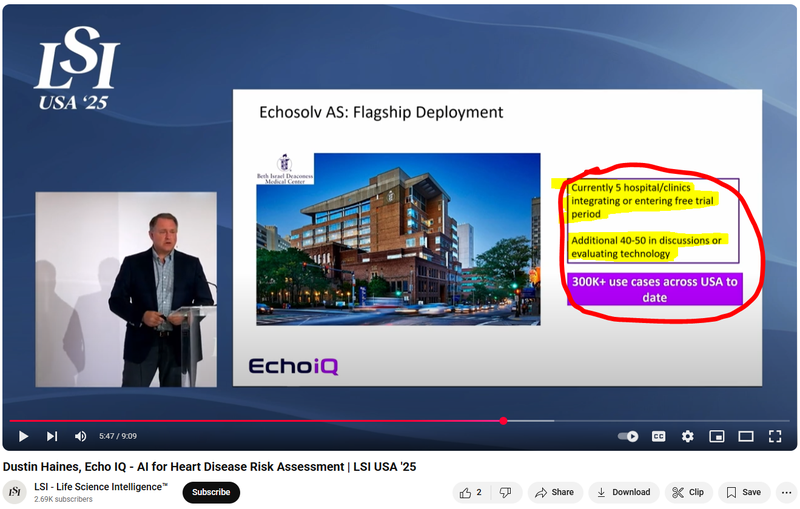
(Watch the full presentation here)
Deals like today’s one also relates to EIQ’s Heart Failure tech…
We mentioned earlier, that integrations are very important because it means EIQ can also distribute newly developed tech into those same networks in the future.
We think this is where EIQ’s Heart Failure tech comes into play.
Heart Failure has a much bigger addressable market relative to AS, potentially worth ~10x more in terms of revenues for EIQ.
Here are some of the market value calculations EIQ has shared about the two in the past:
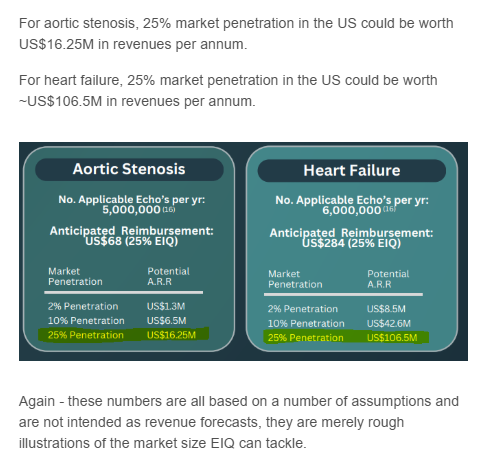
(Source)
EIQ is currently running a clinical validation study for its Heart Failure tech.
The study is a precursor for EIQ’s FDA submission and ultimately FDA clearance which EIQ expects in H2-2025.
FDA clearance for its Heart Failure tech is one of the major catalysts that we think could be a major inflection point for EIQ.
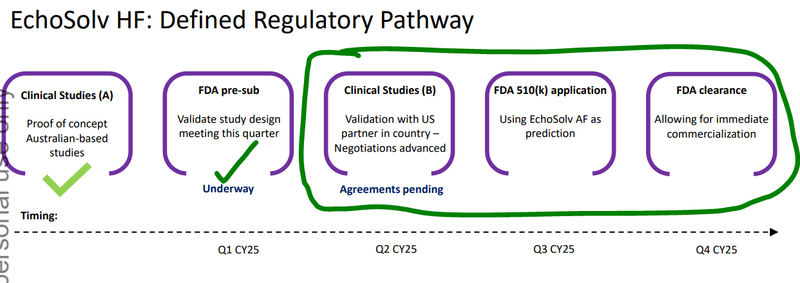
The expected approvals in Q4 are why we think integration newsflow on EIQ’s Aortic Stenosis tech between now and the end of year could be important for EIQ.
What’s next for EIQ?
🔄 Commercialisation updates for Aortic Stenosis AI tech
The key metric we will be tracking in the short term is how many integrations EIQ can secure for its Aortic Stenosis tech.
In the short term we want to see more deals like today’s one.
We also want to see an update on the company’s application to get category III reimbursement codes approved - this is expected sometime in September.
🔄 Heart Failure clinical validation study
We want to see EIQ complete these trials and (hopefully) deliver strong enough results to support an FDA submission for its Heart Failure detection tech.
🔄 Strategic partnership updates
We want to see EIQ advance discussions in this area to help rapidly roll out the company’s tech, grow EIQ’s revenue and build market share.
🔄 Australia and NZ pilot program
EIQ has previously mentioned that this program is being run with a ”leading global structural heart innovation company”.
We want to see some more news on this front because we think it could help advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US.
🔲 Partnership with European reseller to broaden market exposure
We want to see EIQ expand into new markets like Europe, in a previous webinar EIQ said the company was pursuing this opportunity.
This also includes CE Mark and TGA applications so that EIQ can sell into Europe and Australia.






